43 label the chemical equation.
Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. What are the Parts of a Chemical Equation? - Life Persona 2 - Begin by writing the chemical equation in terms of the substances involved: C 2 H 6 T the 2 → CO 2 + H 2 OR We have two carbon atoms left, so we need two molecules of carbon dioxide on the side of the product, so that each side has two carbon atoms. That element is balanced.
Chemical Equation | Reactants And Products In Chemical Reactions A chemical equation consists of reactants, products and an arrow showing the direction of reaction. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation. Law of conservation of mass governs the balancing of a chemical equation.
Label the chemical equation.
Solved Label the parts of the chemical equation with the | Chegg.com Label the parts of the chemical equation with the appropriate descriptions. KOH (aq) + HI (aq) + H, O (1) KI (aq) Answer Bank liquid solid aqueous solution gas reactant chemical change product Question: Label the parts of the chemical equation with the appropriate descriptions. Balancing Equations.pdf - T. Trimpe 2006 2 Mg + O2... (3) Answer the questions related to each chemical formula. Part B: Label the chemical equation using PRODUCT, REACTANTS, SUBSCRIPT, COEFFICIENT, and YIELDS. 2 are in the formula shown? Na = _____ S = _____ O = _____ O 2 What element does the O represent? _____ CO 2 How many atoms of each element are in the formula shown? Al + O2 = Al2O3 - Chemical Equation Balancer Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a Al + b O 2 = c Al 2 O 3. Create a System of Equations. Create an equation for each element (Al, O) where each term represents the number of atoms of the element in each reactant or product. Al: 1 a + 0b = 2 c O: 0a + 2 b = 3 c ...
Label the chemical equation.. Symbols used in Chemical Equations Flashcards | Quizlet used for reversible reactions. +. used to separate two reactants or two products. ∆ (over the reaction arrow), indicates the application of heat to the reactants. ↓. indicates a precipitate formed by a reaction. (aq) designates an aqueous solution; the substance is dissolved H₂O; placed after the chemical formula. Chemical equation - Wikipedia A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction. The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Start by writing the chemical equation in terms of the substances involved: C 2 H 6 + O 2 → CO 2 + H 2 O We have two carbon atoms on the left, so we need two carbon dioxide molecules on the product side, so that each side has two carbon atoms; that element is balanced. What are Chemical Equations? Detailed Explanation, Examples The chemical equations in which electrolytes are represented in the form of dissociated ions are commonly referred to as ionic equations. They are often used to represent the displacement reactions that take place in aqueous mediums. In these reactions, some ions participate in the reaction and some do not.
Chemical Nomenclature and Chemical Formulas - Owlcation It consists of symbols of elements and subscripts which give the number of atoms of each element. Examples: The formula of water is H2O. There are 2 atoms of Hydrogen and 1 atom of oxygen. The formula of glucose is C6H12O6. There are 6 atoms of Carbon, 12 atoms of Hydrogen and 6 atoms of Oxygen. In writing formulas, the total positive charges ... Chemical equations - Formulae and equations - GCSE Chemistry (Single ... Chemical equations. Word equations are useful to show which chemicals react together (the reactants) and which chemicals are produced (the products).Symbol equations allow chemists to work out the ... Label the chemical Equation worksheet Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (0) Download file pdf Embed in my website or blog Answered: Label the following parts of the… | bartleby Label the following parts of the chemical equation below: coefficient, subscript, products, reactants, yields, chemical formula. A. B. A. С. В. C. CaCl, + H,0 Cao + 2HCI D. E. D. E. F. F. The Law of Conservation of Mass states that during a chemical reaction, mass/matter is not Because of this law, there must be exactly Jon each side of a ...
4.1 Writing and Balancing Chemical Equations - Chemistry First, write the unbalanced equation. N2 +O2 N2O5 (unbalanced) N 2 + O 2 N 2 O 5 ( unbalanced) Next, count the number of each type of atom present in the unbalanced equation. Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. How to Write a Chemical Equation (with Pictures) - wikiHow The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. [11] For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2 Switch the ions to build the products. Material Balances — Introduction to Chemical and Biological … Guidelines for solving material balance problems involving multiple species. From pg 76 in Introduction to Chemical Engineering: Tools for Today and Tomorrow (5th Edition):. Determine if species information is required, or if an overall mass balance will suffice. KClO3 = KCl + O2 - Chemical Equation Balancer Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a KClO 3 = b KCl + c O 2. Create a System of Equations. Create an equation for each element (K, Cl, O) where each term represents the number of atoms of the element in each reactant or product. K: 1 a = 1 b + 0c Cl: 1 a = 1 b + 0c O ...
Energy Balances — Introduction to Chemical and Biological … Exercise: Energy balance for a system with a chemical reaction. Suppose the following reaction is carried out in a chemical reactor: \(\ce{A + B -> C}\). The reactor has a single inlet and a single effluent (outlet) and the entire reactor system is at constant density (\(\rho = \SI{0.9}{kg/L}\)).The desired conversion of \(A\) is \(0.8\).. Operating conditions and parameter values
Solved Label the components of a chemical equation. separate | Chegg.com Chemistry Chemistry questions and answers Label the components of a chemical equation. separate mass liquid coefficient gas solution solid reactants layer prod uiets volume gram Na2co, (s) + 2HCl (aq),- ?.co2 (g) + H2O (l) Co2 (g) + H20 ) + 2 Naci (aq)
How do you label a chemical equations? - Answers chemical equations are a formal algebra that permits the calculation of chemical reactions. Why are chemical equations important in chemistry? Chemical equations are graphic representation of ...
What is a Chemical Equation? - Definition & Examples Chemical Equation Analogy. Think of a chemical equation as a mini recipe to make a new substance. To make a beautiful cake, you need to add the correct amounts and proportions of eggs, flour ...
Labeling A Chemical Equation Part 2 - YouTube The instructions for labeling a chemical equation.
Answered: 3. Write a chemical equation using full… | bartleby Write a chemical equation using full… | bartleby. 3. Write a chemical equation using full structures which represents the reaction between acetic acid and sodium hydroxide. 4. Draw the full structure of octyl acetate (a component of orange flavor) and label the "acid" and "alcohol" portions of this ester. 17.
Dimensional analysis - Wikipedia Concrete numbers and base units. Many parameters and measurements in the physical sciences and engineering are expressed as a concrete number—a numerical quantity and a corresponding dimensional unit. Often a quantity is expressed in terms of several other quantities; for example, speed is a combination of length and time, e.g. 60 kilometres per hour or 1.4 kilometres per …
The Balanced Chemical Equation for Photosynthesis In words, the equation may be stated as: Six carbon dioxide molecules and six water molecules react to produce one glucose molecule and six oxygen molecules . The reaction requires energy in the form of light to overcome the activation energy needed for the reaction to proceed. Carbon dioxide and water don't spontaneously convert into glucose ...
Chemical Equations Flashcards | Quizlet Chemical Equation symbolic representation of a chemical reaction, will show the same # of each type of atom on each side of the equation. ex. Na + Cl2 -> NaCl Chemical Formula symbolic representation of of an element or compound. ex. NaCl (table salt) Chemical Reaction process in which bonds between atoms are broken and new bonds are formed.


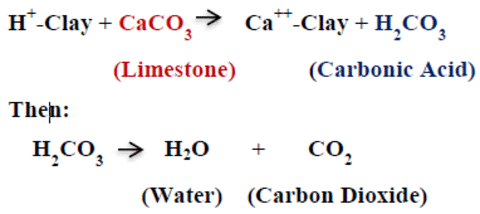



Post a Comment for "43 label the chemical equation."