44 label each stereogenic center as r or s.
Journal of the American Chemical Society | Vol 143, No 33 The deposition of Cs on Cu/ZnO(0001̅) is found to greatly facilitate methanol synthesis and enable the ethanol synthesis from CO2 hydrogenation. The synergy among Cs, Cu, and ZnO at the interface plays an essential role, being able to promote the CO2 and tune the selectivity toward methanol and ethanol. View the article. Stereoisomers Types & Examples - Study.com Feb 26, 2022 · Draw all possible stereoisomers of 1,3-dichlorocyclopentane, and label each structure as A, B etc. a) Label all chiral C's, b) write "chiral" by chiral isomers, c) write "meso" if appropriate, a
SOLVED:Label each stereogenic center as R or S. this question asks us to aside, as are our configuration for Glucose s. So this is me glucose. Um, again gonna start by assigning the, um ah. Parity of the group sold is this one? This is two. This is three and Hodgins on this side, so we have to reverse the order. So 123 is cattle. Clockwise prefers is, huh? This is one again. Appears to marry is three.

Label each stereogenic center as r or s.
Solved: Chapter 5 Problem 47P Solution - Chegg Rule 3: If the first atom of each substituent is same then give priority to the second atom in each substituent. This process continues to the third and fourth atom until the rule difference is reached. Rule 4: If the substituents have multiple bonds, the multiple bonded atoms are considered as same number of single bonded atoms. Answered: Write the configuration of two… | bartleby 2. Assign the absolute configuration for each chiral carbon by writing the carbon number followed by R or S enclosed in parenthesis (e.g. 2R, 3S, 4R, 5S). Answers must be written on the right side of the Fischer projection aligned with the corresponding chiral carbon. 3. Answered: Label each stereogenic center as R or S… | bartleby The stereogenic center in the given compound has to be mentioned, Q: C) Label each asymmetric carbon in the compound below as R or S. H3C CH2CH2CI H3C CHO ⊙ Draw semicircle goes from 1→2→3 then check clockwise/anticlockwise rotation and 4th priority…
Label each stereogenic center as r or s.. Caffeine - Wikipedia It is a regulatory requirement that the label of most prepackaged foods must declare a list of ingredients, including food additives such as caffeine, in descending order of proportion. However, there is no regulatory provision for mandatory quantitative labeling of caffeine, (e.g., milligrams caffeine per stated serving size). Assign $R,S$ designations to each stereogenic center in glucose. VIDEO ANSWER: this question asks us to aside, as are our configuration for Glucose s. So this is me glucose. Um, again gonna start by assigning the, um ah. Par… How to Assign R / S Configurations to Chiral Centers - dummies Step 1: Prioritizing the substituents. The first step is to prioritize all the substituents from one to four. Bromine is the atom with the largest atomic number, so this substituent is given the highest priority; hydrogen has the smallest atomic number, so it's given the lowest priority. Chlorine gets the number-two priority because it has a ... 4.4: Labeling Stereogenic Centers with R or S - Chemistry LibreTexts Stereocenters are labeled R or S The "right hand" and "left hand" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S. Consider the first picture: a curved arrow is drawn from the highest priority ( 1) substituent to the lowest priority (4) substituent.
Solved Label each stereogenic center as R or S. | Chegg.com Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 96% (80 ratings) Transcribed image text: Label each stereogenic center as R or S. Previous question Next question. Draw the eight constitutional isomers having the molecular f | Quizlet C5H11Cl. a. Give the IUPAC name for each compound (ignoring R and S designations). b. Label any stereogenic centers. c. For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S. Solved: Label each stereogenic center as R or S. | Chegg.com Organic Chemistry (4th Edition) Edit edition Solutions for Chapter 28 Problem 3P: Label each stereogenic center as R or S. … Solutions for problems in chapter 28 1P SOLVED:Label each stereogenic center as R or S. - Numerade let's label each stereo centers are s for part A iodine has the highest priority than the Ethel on the metal than hydrogen. But the lowest priority back, which is hydrogen. And if we move around the ring me of iodine, Ethyl carbon. This is the counter clockwise direction.
Chiral drugs - Wikipedia In this approach: identify the chiral center, label the four atoms directly attached to the stereogenic center in question, assign priorities according to the sequence rule ( from 1 to 4), rotate the molecule until the lowest priority (number 4) substituent is away from the observer/viewer, draw a curve from number 1 to number 2 to number 3 ... SOLVED:Label each stereogenic center as R or S. - Numerade question as us to label each steer. Oh, center Azarias are so wonderful that we need to notice the lowest of the highest number in this case. Most of most of the cases hydrogen. If it's this line, then we keep the order. And if this light, then we reverse the artist. Cobalt(III) Carbene Complex with an Electronic Excited-State ... Many organometallic iridium(III) complexes have photoactive excited states with mixed metal-to-ligand and intraligand charge transfer (MLCT/ILCT) character, which form the basis for numerous applications in photophysics and photochemistry. Cobalt(III) complexes with analogous MLCT excited-state properties seem to be unknown yet, despite the fact that iridium(III) and cobalt(III) can adopt ... R/S - Two Stereogenic Centers R/S Naming Diastereoisomerism Meso Compounds Today, we'll look at naming compounds with stereocenters, and then we'll examine the complications which arise when a molecule has more than one stereocenter in it. First, though, let's look at a property in which one enantiomer differs from another. Enantiomers are alike in all respects but one.
Biosynthesis of cyanobacterin, a paradigm for furanolide core ... May 26, 2022 · Thermal cycling was performed in a Bio-Rad T100 thermal cycler and began with an initial denaturation cycle of 98 °C for 2 min, followed by 30 cycles of DNA denaturation at 98 °C for 20 s ...
7.2: Naming chiral centers: the R and S system The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations ' R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
Answered: Label each stereogenic center as R or S… | bartleby The stereogenic center in the given compound has to be mentioned, Q: C) Label each asymmetric carbon in the compound below as R or S. H3C CH2CH2CI H3C CHO ⊙ Draw semicircle goes from 1→2→3 then check clockwise/anticlockwise rotation and 4th priority…
Answered: Write the configuration of two… | bartleby 2. Assign the absolute configuration for each chiral carbon by writing the carbon number followed by R or S enclosed in parenthesis (e.g. 2R, 3S, 4R, 5S). Answers must be written on the right side of the Fischer projection aligned with the corresponding chiral carbon. 3.
Solved: Chapter 5 Problem 47P Solution - Chegg Rule 3: If the first atom of each substituent is same then give priority to the second atom in each substituent. This process continues to the third and fourth atom until the rule difference is reached. Rule 4: If the substituents have multiple bonds, the multiple bonded atoms are considered as same number of single bonded atoms.
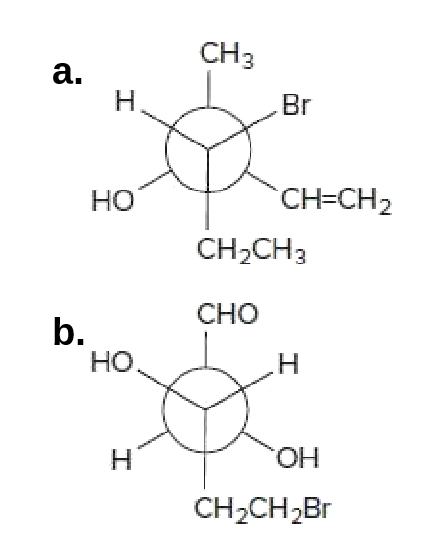

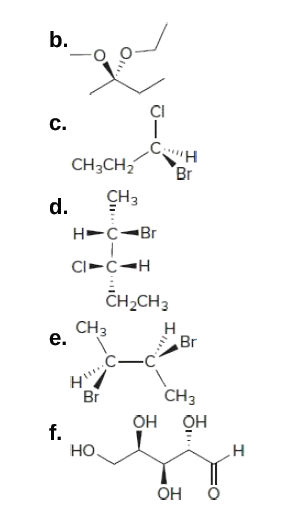


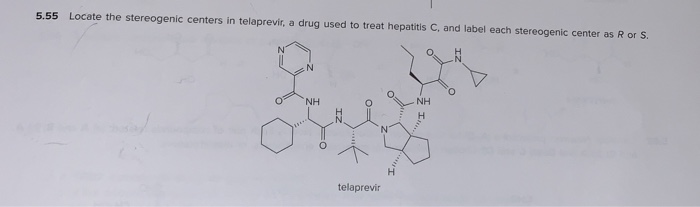
Post a Comment for "44 label each stereogenic center as r or s."