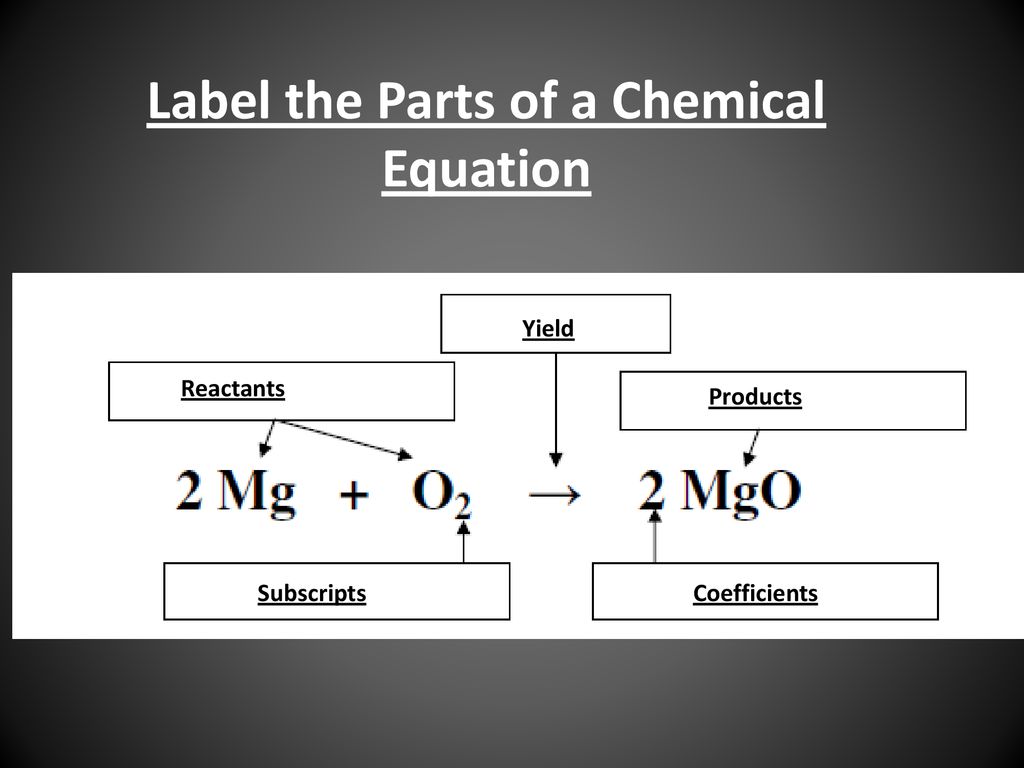
45 label the reactants and products in the chemical reaction
Study Guide 2.4- 2.5 Answers reactant product activation energy. MAIN IDEA: Bonds break and form during chemical reactions. 1. Label the reactants and products in the chemical reaction ... H2 + Cl2 = HCl - Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored.
Answered: For the following reaction: & e H 1.… | bartleby 01.08.2022 · Q: Predict the products (A and B) of Reaction 1, determine the reactants (D and E) and the necessary… A: Click to see the answer Q: A sample of solid NH 4NO 3 was placed in an evacuated container and then heated so that it…

Label the reactants and products in the chemical reaction
Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in ... Label each reactant and product in the given chemical reaction ... In a chemical equation, reactants and products are present at the left-hand side and right-hand side of the arrow respectively. In the given... Reactants and Products ( Read ) | Chemistry | CK-12 Foundation Reactants and products in chemical reactions and the relationship between reactants and products. Click Create Assignment to assign this modality to your LMS. ... The materials involved in a chemical reaction. % Progress . MEMORY METER. This indicates how strong in your memory this concept is. Practice. Preview; Assign Practice; Preview. Progress %
Label the reactants and products in the chemical reaction. 8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula So if the concentration of one (or more) of the reactants or products is increased the equilibrium will shift to decrease the concentration. Or if the temperature is decreased the equilibrium will shift to increase the temperature by favouring the exothermic reaction. Le Chatelier's principle is … 2.4 Flashcards | Quizlet A chemical reaction changes reactants into products. Write your own analogy to remember the meaning of activation energy. The energy it takes to get out of bed in the morning before you can start your day. The term "equilibrium" is based on two Latin roots that mean "equal" and "balance." For the following chemical reaction, label the reactants and - Quizlet ... to the following textbook question: For the following chemical reaction, label the reactants and products, and then balance the chemical equation. Real-time polymerase chain reaction - Wikipedia A real-time polymerase chain reaction (real-time PCR, or qPCR) is a laboratory technique of molecular biology based on the polymerase chain reaction (PCR). It monitors the amplification of a targeted DNA molecule during the PCR (i.e., in real time), not at its end, as in conventional PCR. Real-time PCR can be used quantitatively (quantitative real-time PCR) and semi-quantitatively …
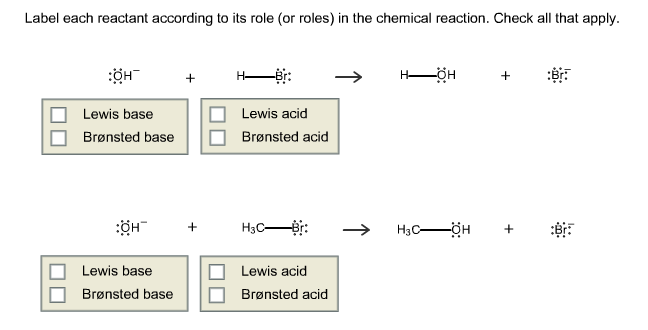
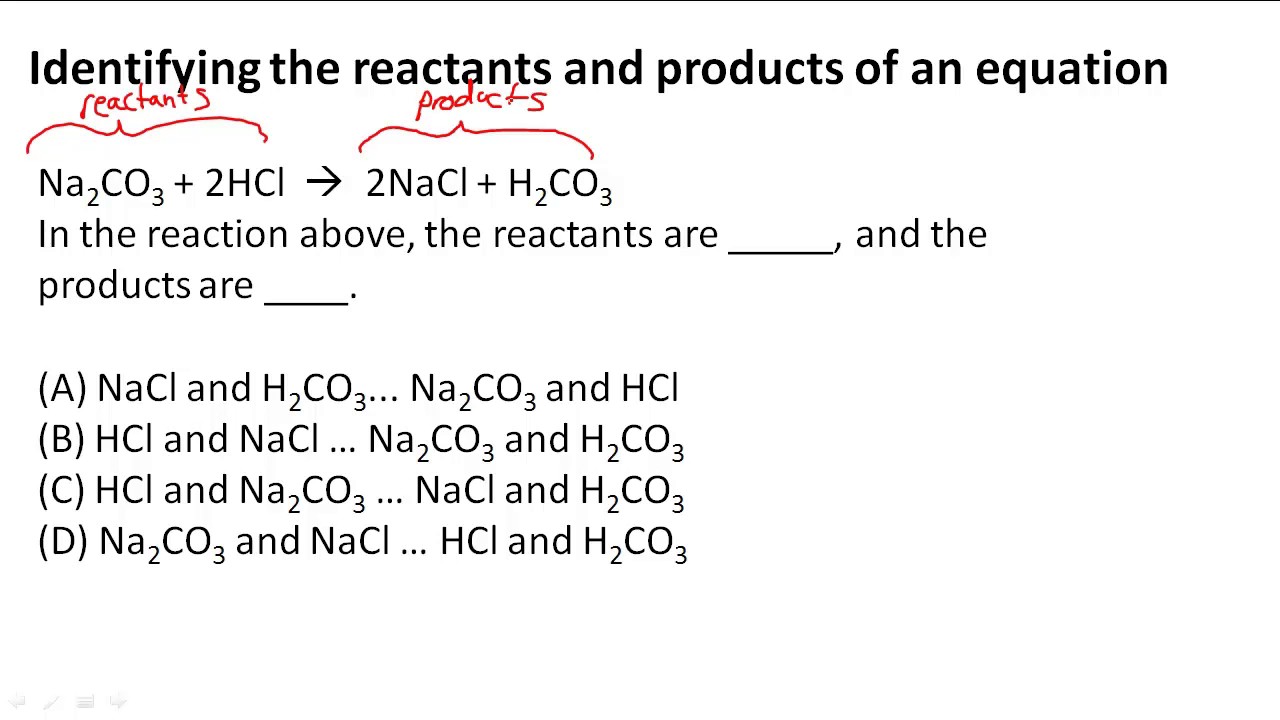
How would you label each formula in the chemical equation ... - Socratic Explanation: The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron, F e +S → F eS Iron and sulfur are the reactants, and iron sulfide is the product. Answer link Answered: label the reactants and the products… | bartleby A: Q: Predict the products of the following: benzoic acid + HNO3/H2SO4 Br CH3 + HNO3/H2S04 → phenol +…. A: Detail mechanistic pathway is given below to find out the product for every reaction. Q: clearly indicate your assignments of all pro 1.37 17. Draw the structures of Compound 17a. and 17b.…. A: Solved Label each reactant and product in this reaction as a - Chegg Label each reactant and product in this reaction as a Bronsted acid or base. Classify each of the following reactants and products as an acid or base according to the Bronsted theory CF3COOH + H2,0 H3O+ + CF3COO- This problem has been solved! See the answer O. Chem Show transcribed image text Expert Answer 100% (90 ratings) 2.5: Reaction Rate - Chemistry LibreTexts 17.06.2022 · Definition of Reaction Rate. The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. The speed of a chemical reaction may be defined as the change in concentration of a substance divided by the time interval during which this change is observed:
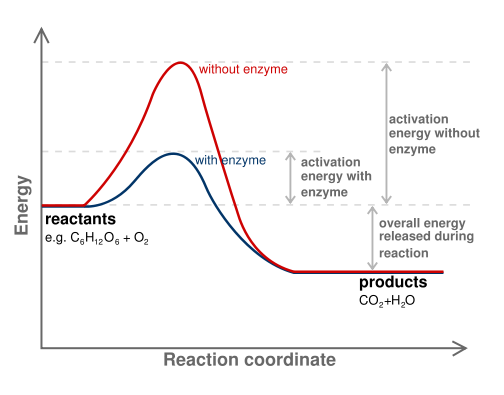
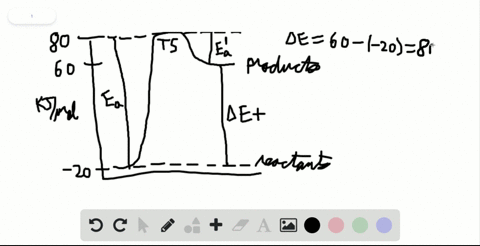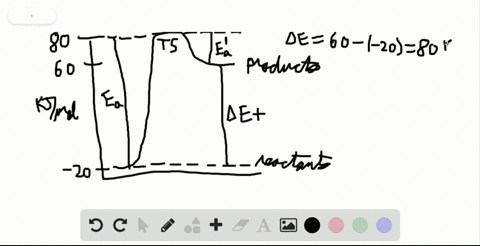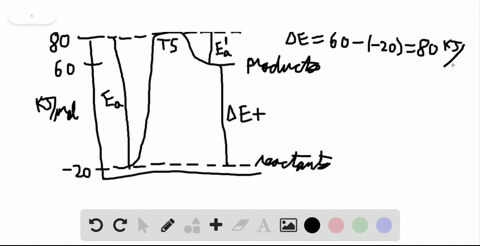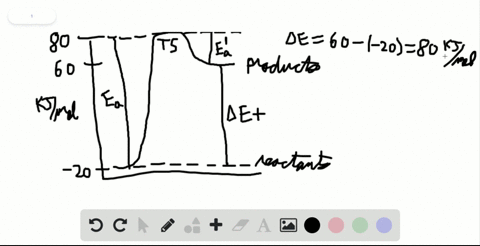
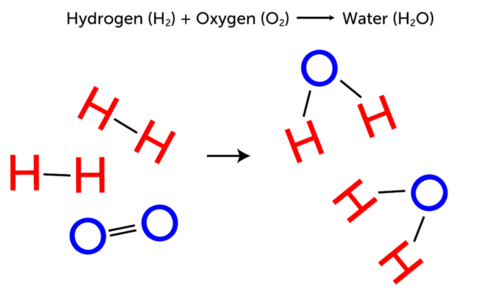
7.2 Reactants and products | Chemical reactions | Siyavula REACTANTS (before the reaction) → PRODUCTS (after the reaction) Do you see how the atoms have rearranged? This means a chemical reaction has taken place. Label the diagram with 'reactants' and 'product'. The reaction between carbon and oxygen takes place when we burn coal. Coal is carbon and when it burns in oxygen gas, carbon dioxide is formed. Exothermic and endothermic reactions - AQA - BBC Bitesize It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The energy level decreases in an exothermic reaction. This is because energy is... C3H8 + O2 = CO2 + H2O - Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. What Is the Chemical Equation for Cellular Respiration? Dec 13, 2021 · The reactants in a chemical reaction are the substances that are used in the reaction and are turned into something else. Reactants show up on the left side of the equation before the arrow ...
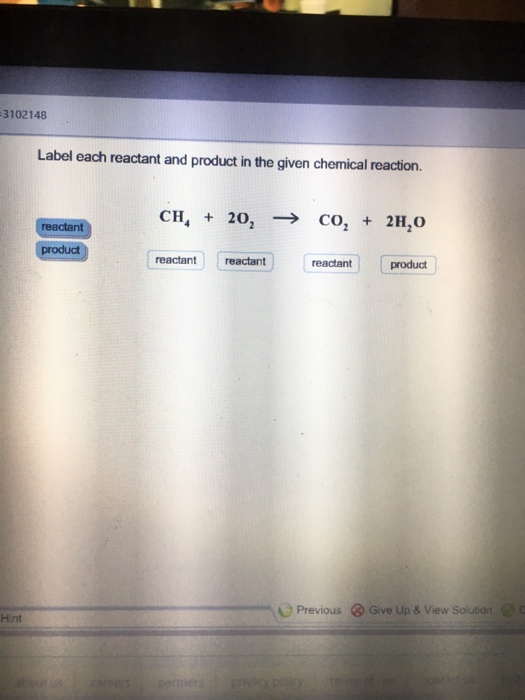
Solved 1. Label each reactant and product in the given | Chegg.com 1. Label each reactant and product in the given chemical reaction. CH4 + 2O2 CO2+ 2H2OCH4 + 2O2 CO2 + 2H2O Answer Bank: Product/Reactant 2. Use the law of conservation of mass to answer the questions. Consider a hypothetical reaction in which A and B are reactants and C and D are products.
What are the reactants in the chemical reaction CO2 H2O H2CO3? - Answers The compound which reacts in chemical reaction is know as reactants. products mean the results what we yields from reactants. example: c + o2 -> co2 here c and o2 are reactants and co2 is product.
SOLVED:Label the reactants and products 0 the enthalpy diagrams for ... Hello here we have to label reactions and the products on the internal P. Tech er diagram for his reaction. Let's start with an atomic reaction. So in an ender thermic reaction the the age yes positive. That's why products have higher entropy and reactors will be lower in. Meanwhile, for the exit ceramic reaction it's the opposite. So for the next attorney reaction the issue is negative.
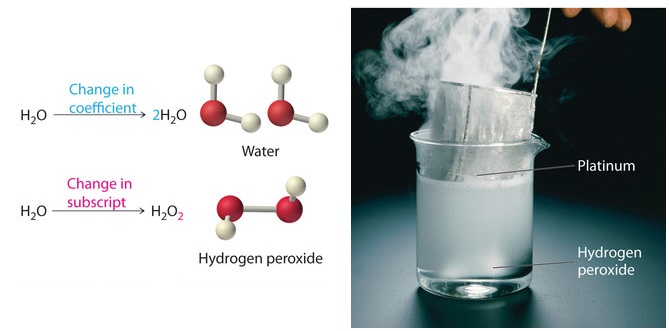
Controlling the Amount of Products in a Chemical Reaction Point out that the type and number of atoms in the reactants and in the products are exactly the same. This is an important concept in chemistry: In a chemical reaction, all the atoms in the reactants end up in the products. When an equation of a chemical reaction is written, it is "balanced" to show this.
Temperature and the Rate of a Chemical Reaction - Middle … Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the temperature increases the average speed of the reactant molecules. As more molecules move faster, the number of molecules moving fast enough to react increases, which results in faster formation of products.
Reactants and Products in Chemical Reactions - dummies Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses). In this reaction, all reactants and products are invisible. The heat being evolved is the clue that tells you a reaction is taking place.
15.2: The Equilibrium Constant (K) - Chemistry LibreTexts Aug 14, 2020 · For the general reaction \(aA+bB \rightleftharpoons cC+dD\), in which all the components are gases, the equilibrium constant expression can be written as the ratio of the partial pressures of the products and reactants (each raised to its coefficient in the chemical equation):
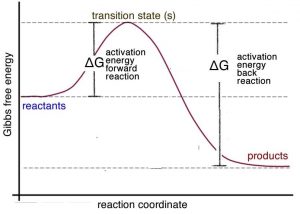
Label the x and y axes the reactants and the products Label the x- and y-axes, the reactants, and the products on the diagram. Label the total change in enthalpy (ΔH) and activation energy (Ea) on your diagram. Make sure correct units are included.
Chemical reactions - Department of Education and Training 18.08.2020 · When a chemical reaction does take place, they believe that one or other of the reactants is simply modified; it hasn't really changed. For example, students consider that rust is still iron/steel; it has just gone brown. Similarly, rust flaking off is usually not noticed – it is thought that the iron just disappears. Gas bubbles that are frequently produced when a tablet …
Label each reactant and product in the given chemical reaction. Reactants are the substances on left side of the reaction which are taken to make new substances. CH4 C H 4 - Methane ( prefix meth- for one carbon ...
Radioactive tracer - Wikipedia A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form …
CK12-Foundation A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products. A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
Reactants Products Chemical Reaction Teaching Resources | TpT A chemical reactions activity where students measure the amount of carbon dioxide that is produced from the vinegar-baking soda reaction. The reaction is an excellent example to use when teaching about the reactants and products of chemical equations. The activity is completely described and directions to measure the carbon dioxide are given.
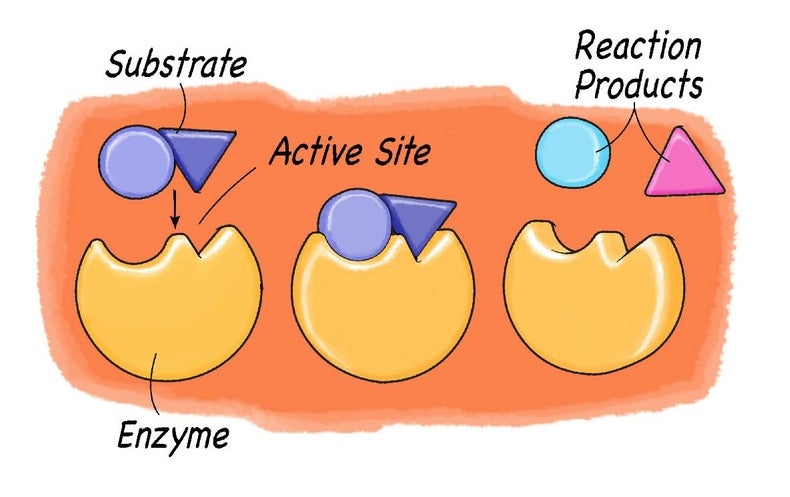
8.2 How do we represent chemical reactions? - Siyavula The materials we start with are called reactants, and the new materials that form are called products. During a chemical reaction, atoms are rearranged. This requires that bonds are broken in the reactants and new bonds are formed in the products. In this chapter we are going to build on these ideas. We will focus on two things:
Combustion Reaction Formula and Products - Study.com The combustion reaction formula shows the reactants and products of the combustion reaction. The combustion reaction formula can be written with the hydrocarbon and oxygen on the left side; carbon ...
Chemical Equation | Reactants And Products In Chemical Reactions - BYJUS The representation of a chemical reaction in the form of symbols (substances) is known as chemical equation. A chemical equation consists of reactants, products and an arrow showing the direction of reaction. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation.
Analyzing Chemical Reactions Student Guide - Brainly.com a) Draw Lewis dot diagrams of the reactants and products on the paper with your chemical equation. b) Select a different color to use for each element in the chemical reaction. Use your chosen color for the element's symbol and for its valence electrons. c) Label these diagrams "Lewis Dot Diagrams."
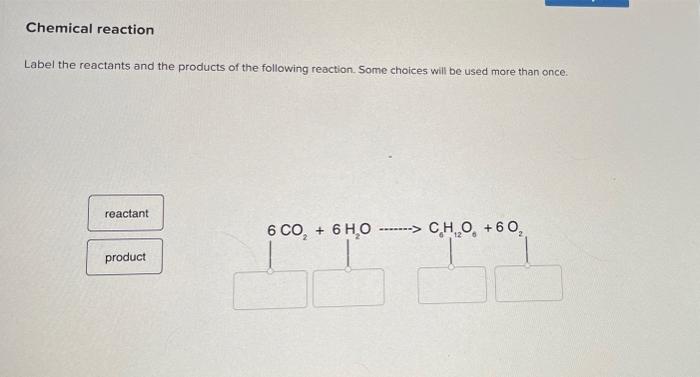
Solved Chemical reaction Label the reactants and the | Chegg.com Question: Chemical reaction Label the reactants and the products of the following reaction. Some choices will be used more than once reactant 6 CO.
Reactants and Products Flashcards | Quizlet 2H2+O2 - 2H2O represents the law of conservation of mass True Why does a chemical reaction need to be balanced? So all of the atoms are present in both the product and the reactant, and so no atoms magically appear or disappear as a result of the reaction. (Two answers are correct)
Reactants and Products - GeeksforGeeks Any chemical reaction involves both reactants and products. The wax of a candle and the oxygen in the air are reactants in a combustion reaction. Carbon dioxide and water vapour are the byproducts. When methane gas is burned, the reactants are methane (CH 4) and oxygen in the air (O 2 ).
7.2 Reactants and products | Chemical reactions - Siyavula Do you see how the atoms have rearranged? This means a chemical reaction has taken place. Label the diagram with 'reactants' and 'product'.
5 types of Chemical Reactions with Chemical Reaction examples The changes which occur within the reactants during a chemical reaction determine the types of chemical reaction and the nature of products. Based on this rule, chemical reactions are often divided into five common types. Most of the products we get in our daily lives come into existence by going through one of these five reactions.
reactants and products | Science Quiz - Quizizz Identify the reactants and products in the following chemical reaction: CH4 + 2O2 → CO2 + 2H2O. answer choices. The reactants are CO2 and 2O2. The products are CH4 and 2H2O. The reactants are CO2 and 2O2. The products are CH4 and 2H2O. The reactants are CH4 and 2H2O. The products are CO2 and 2O2.
Laebling A Chemical Equation Part I - YouTube Labeling chemical equations and identifing reactants, yield and products.
The Reactants And Products Of Cellular Respiration Cellular respiration is the process responsible for converting chemical energy, and the reactants/products involved in cellular respiration are oxygen, glucose (sugar), carbon dioxide, and water. While the exact steps involved in cellular respiration may vary from species to species, all living organisms perform some type of cellular respiration.
What are examples of reactants and products in chemistry? Originally Answered: What are the examples of reactants and product in chemistry? 1. H2 + O2-->2H2O Here h2 and o2 are reactants. and h2o is product 2. FeO2 + SO2-->FeSO4 Here again FeO2 and SO2 are the reactants while FeSO4 is the product Promoted by The Penny Hoarder Kyle Taylor Founder at 2010-present Quora User
Photosynthesis: Reactants and Products - Visible Body You can think of the reactants as the ingredients for preparing a meal and the products as the different dishes in that meal. With that in mind, let's take a look at the chemical equation for photosynthesis: Sunlight + 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2. CO 2 = carbon dioxide. H 2 O = water. C 6 H 12 O 6 = glucose.
[Solved] How does knowing the reactants and products help you classify ... Knowing the reactants and products you can tell to which one of those four classifications a reaction pertains. When two reactants combine into a single product, it is a combination (syntheisis) reaction. When a single reactant yields two or more products, it is a decomposition reaction.
Reactants and Products | Chemistry for Non-Majors | | Course Hero A reactant is a substance that is present at the start of a chemical reaction. The substance (s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
networkx - Python program to map a series of chemical reactions with ... Could anyone advise me on the best way to proceed with this, I need a function that will identify the common species in reactants and products in all reactions and create a single node for each unique species and create one line from each reactant to each product for each reaction. Any help would be hugely appreciated. Thank you very much
Reactants and Products ( Read ) | Chemistry | CK-12 Foundation Reactants and products in chemical reactions and the relationship between reactants and products. Click Create Assignment to assign this modality to your LMS. ... The materials involved in a chemical reaction. % Progress . MEMORY METER. This indicates how strong in your memory this concept is. Practice. Preview; Assign Practice; Preview. Progress %
Label each reactant and product in the given chemical reaction ... In a chemical equation, reactants and products are present at the left-hand side and right-hand side of the arrow respectively. In the given...
Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in ...









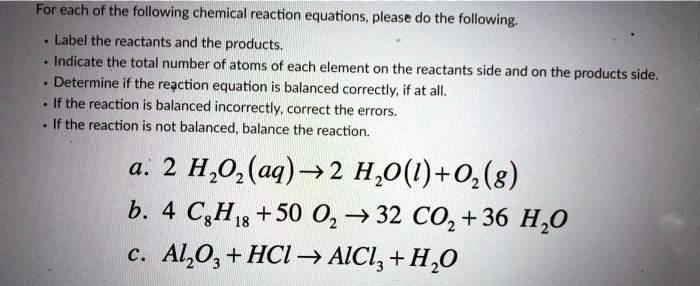

















Post a Comment for "45 label the reactants and products in the chemical reaction"